What Is Another Name For Heat Equation . Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c °c. This equation is also known as the diffusion equation. Get the heat definition, formulas, and example calculations. Solving partial differential equations (pdes) by computer, particularly the heat equation. There is a difference between thermal energy and heat. The symbol c stands for the. While thermal energy refers to the motion of. Another word for thermal energy is heat energy, not to be confused with heat. We can use the heat capacity to determine the heat released or absorbed by a material using the following formula: Q = m × c × δ t where m is. Learn about heat in thermodynamics in physics and chemistry. Consider a liquid in which a dye is being diffused through the liquid. In equation form, heat capacity c is c = m c. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature.
from smartclass4kids.com
Get the heat definition, formulas, and example calculations. Another word for thermal energy is heat energy, not to be confused with heat. Learn about heat in thermodynamics in physics and chemistry. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c °c. We can use the heat capacity to determine the heat released or absorbed by a material using the following formula: Q = m × c × δ t where m is. There is a difference between thermal energy and heat. While thermal energy refers to the motion of. In equation form, heat capacity c is c = m c. The symbol c stands for the.
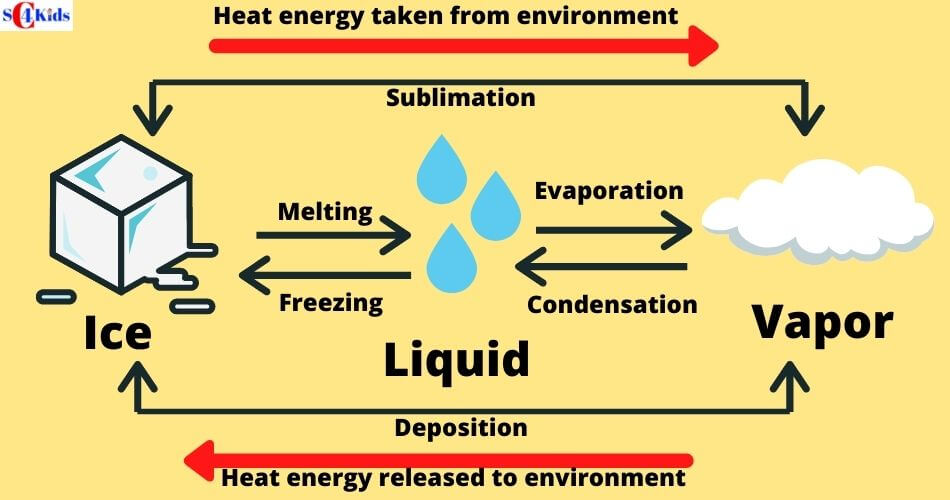
Changing States of Matter Solid, Liquid,Gas, Phase Change
What Is Another Name For Heat Equation Another word for thermal energy is heat energy, not to be confused with heat. The symbol c stands for the. Q = m × c × δ t where m is. This equation is also known as the diffusion equation. While thermal energy refers to the motion of. There is a difference between thermal energy and heat. We can use the heat capacity to determine the heat released or absorbed by a material using the following formula: Get the heat definition, formulas, and example calculations. Learn about heat in thermodynamics in physics and chemistry. Another word for thermal energy is heat energy, not to be confused with heat. In equation form, heat capacity c is c = m c. Consider a liquid in which a dye is being diffused through the liquid. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c °c. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Solving partial differential equations (pdes) by computer, particularly the heat equation.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change What Is Another Name For Heat Equation Another word for thermal energy is heat energy, not to be confused with heat. Get the heat definition, formulas, and example calculations. Q = m × c × δ t where m is. We can use the heat capacity to determine the heat released or absorbed by a material using the following formula: Heat capacity is the amount. What Is Another Name For Heat Equation.
From www.hotelsrate.org
Chemical Thermodynamics Equations Sheet Diy Projects What Is Another Name For Heat Equation Another word for thermal energy is heat energy, not to be confused with heat. We can use the heat capacity to determine the heat released or absorbed by a material using the following formula: The symbol c stands for the. Q = m × c × δ t where m is. There is a difference between thermal energy. What Is Another Name For Heat Equation.
From spectacularsci.com
What Are The 3 Types of Heat Transfer? Spectacular Science What Is Another Name For Heat Equation Get the heat definition, formulas, and example calculations. Another word for thermal energy is heat energy, not to be confused with heat. The symbol c stands for the. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c °c. Q = mcδt, where q is the symbol for heat transfer (“quantity of. What Is Another Name For Heat Equation.
From www.vulcanic.com
To calculate heating power Vulcanic What Is Another Name For Heat Equation This equation is also known as the diffusion equation. Another word for thermal energy is heat energy, not to be confused with heat. There is a difference between thermal energy and heat. While thermal energy refers to the motion of. Get the heat definition, formulas, and example calculations. We can use the heat capacity to determine the heat released or. What Is Another Name For Heat Equation.
From elainegokemahoney.blogspot.com
Calcium Carbonate on Heating Balanced Equation What Is Another Name For Heat Equation Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Q = m × c × δ t where m is. Learn about heat in thermodynamics in physics and chemistry. Another word for thermal energy is heat energy, not to. What Is Another Name For Heat Equation.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential What Is Another Name For Heat Equation Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. While thermal energy refers to the motion of. Consider a liquid in which a dye is being diffused through the liquid. Another word for thermal energy is heat energy, not to be. What Is Another Name For Heat Equation.
From www.youtube.com
Symbols for Chemical reactions YouTube What Is Another Name For Heat Equation Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c °c. While thermal energy refers to the motion of. This equation is also known as the diffusion equation. Get the heat definition, formulas, and example calculations. Solving partial differential equations (pdes) by computer, particularly the heat equation. We can use the heat. What Is Another Name For Heat Equation.
From studylib.net
Dougherty Valley HS AP Chemistry Name Heating Curve Practice What Is Another Name For Heat Equation Q = m × c × δ t where m is. Another word for thermal energy is heat energy, not to be confused with heat. Learn about heat in thermodynamics in physics and chemistry. This equation is also known as the diffusion equation. While thermal energy refers to the motion of. Q = mcδt, where q is the. What Is Another Name For Heat Equation.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID632515 What Is Another Name For Heat Equation Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c °c. This equation is also known as the diffusion equation. We can use the heat capacity to determine the heat released or absorbed by a material using the following formula: In equation form, heat capacity c is c = m c. While. What Is Another Name For Heat Equation.
From studylib.net
Physical Science II Name Heat Review What Is Another Name For Heat Equation Consider a liquid in which a dye is being diffused through the liquid. In equation form, heat capacity c is c = m c. Get the heat definition, formulas, and example calculations. Another word for thermal energy is heat energy, not to be confused with heat. Learn about heat in thermodynamics in physics and chemistry. There is a difference between. What Is Another Name For Heat Equation.
From swiser.github.io
Function Name heat_equation_explicit_euler MATH5620 What Is Another Name For Heat Equation Consider a liquid in which a dye is being diffused through the liquid. The symbol c stands for the. There is a difference between thermal energy and heat. We can use the heat capacity to determine the heat released or absorbed by a material using the following formula: Q = m × c × δ t where m . What Is Another Name For Heat Equation.
From kolblabs.com
Heating of calcium carbonite in Chemical Reactions and Equations What Is Another Name For Heat Equation Q = m × c × δ t where m is. There is a difference between thermal energy and heat. Consider a liquid in which a dye is being diffused through the liquid. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the. What Is Another Name For Heat Equation.
From gmtacademy.blogspot.com
Greater Minds Tutors Chemistry What Is Another Name For Heat Equation Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c °c. There is a difference between thermal energy and heat. While thermal energy refers to the motion of. The symbol c stands for the. In equation form, heat capacity c is c = m c. We can use the heat capacity to. What Is Another Name For Heat Equation.
From www.youtube.com
ELECTRICAL HEATING PART 02 DESIGN OF HEATING ELEMENT AND What Is Another Name For Heat Equation Learn about heat in thermodynamics in physics and chemistry. The symbol c stands for the. Another word for thermal energy is heat energy, not to be confused with heat. Get the heat definition, formulas, and example calculations. This equation is also known as the diffusion equation. In equation form, heat capacity c is c = m c. Q = m. What Is Another Name For Heat Equation.
From buycanonpixma.blogspot.com
Derive Raoult's Law Equation DIFFRACTIONOF XRAYS THROUGH CRYSTALS What Is Another Name For Heat Equation While thermal energy refers to the motion of. Learn about heat in thermodynamics in physics and chemistry. We can use the heat capacity to determine the heat released or absorbed by a material using the following formula: Solving partial differential equations (pdes) by computer, particularly the heat equation. Q = m × c × δ t where m . What Is Another Name For Heat Equation.
From www.coursehero.com
[Solved] What is another name for heat equation? a) Induction equation What Is Another Name For Heat Equation Learn about heat in thermodynamics in physics and chemistry. This equation is also known as the diffusion equation. Get the heat definition, formulas, and example calculations. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Q = m × c ×. What Is Another Name For Heat Equation.
From www.linquip.com
Heat Pump Efficiency Equation & Formula Linquip What Is Another Name For Heat Equation Learn about heat in thermodynamics in physics and chemistry. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c °c. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. There is a difference. What Is Another Name For Heat Equation.
From www.tessshebaylo.com
Energy Balance Equation For Heat Transfer Tessshebaylo What Is Another Name For Heat Equation In equation form, heat capacity c is c = m c. This equation is also known as the diffusion equation. Consider a liquid in which a dye is being diffused through the liquid. While thermal energy refers to the motion of. Another word for thermal energy is heat energy, not to be confused with heat. Heat capacity is the amount. What Is Another Name For Heat Equation.